Femogesal 21 Tablets
8 products sold in last 10 hours
Selling fast! Over 15 people have in their cart
20 people are viewing this right now
- Estimated Delivery : Same day
- Free Shipping & Returns : On all orders over 200 EGP




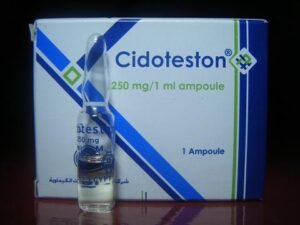






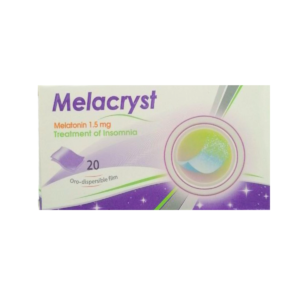
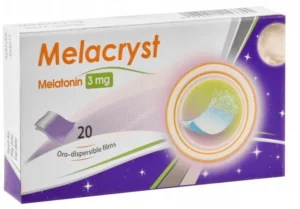

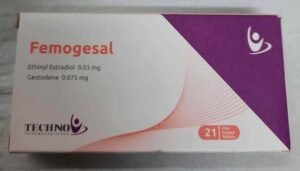
Reviews
There are no reviews yet.