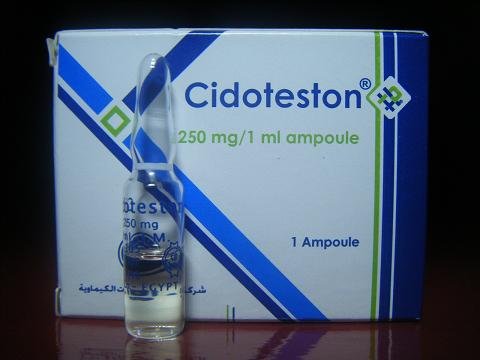
Cidoteston 250 mg amp
- Estimated Delivery : Same day
- Free Shipping & Returns : On all orders over 200 EGP
Medication Name: Cidoteston 250 mg ampoule
Active Ingredient: Testosterone cypionate (250 mg per 1 ml ampoule)
Drug Class: Androgen / Anabolic steroid
Indication:
Used for testosterone replacement therapy in males with confirmed hypogonadism (low testosterone levels). It helps restore normal testosterone levels and alleviate related symptoms such as:
-
Fatigue and low energy
-
Decreased libido or sexual performance
-
Mood changes or depression
-
Loss of muscle mass or strength
Dosage and Administration:
-
Usually 1 ampoule (250 mg) injected intramuscularly every 2 to 3 weeks, depending on individual testosterone levels and medical evaluation.
-
Injection should be deep IM, commonly in the gluteal muscle.
-
The dosing interval may be adjusted by the physician based on blood testosterone levels and response.
Side Effects:
-
Acne or oily skin
-
Increased body hair
-
Fluid retention
-
Mood changes or aggression
-
Gynecomastia (breast enlargement)
-
Changes in cholesterol levels
-
Possible decrease in sperm count with prolonged use
Warnings and Precautions:
-
Should not be used in men with prostate or breast cancer.
-
Requires regular monitoring of testosterone levels, liver function, and hematocrit.
-
Use cautiously in patients with heart, liver, or kidney disease.
-
Misuse (for bodybuilding or performance enhancement) can lead to serious health risks.
Storage:
Store below 25°C, away from direct light and moisture.









Reviews
There are no reviews yet.